
Book M1: 2021 Mini Pocket-Sized (3" x 5") ICH Guidelines for GCP (E6) – Clinical Research Resources, LLC

REFRESHER: ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Fiona Stanley Hospital) - RETProgram

ICH GCP Guidelines E6 Revision, R2 Addendum - Changes Impacting Sponsors-CRO-Sites - Webinar Compliance

ICH Public Web Conference on ICH E6 Guideline for Good Clinical Practice - Update on Progress | Pharmaceuticals and Medical Devices Agency

Clinicubes on Twitter: "✔️ICH GCP E6 (R2) FDA Guidance has been released. ❗An important read for all clinical research professionals: ➡️https://t.co/ok3QmHsaWI #ClinicalTrials #GCP #GxP #Trals #FDA #clinicalresearch #ICHGCPE6 https://t.co ...

Guideline for good clinical practice E6(R2) / guideline-for-good-clinical- practice-e6-r2.pdf / PDF4PRO

Final ICH GCP E6 R2 Addendum: Overview of Changes Impacting Sponsors/CROs/ Clinical Investigator/Site - YouTube










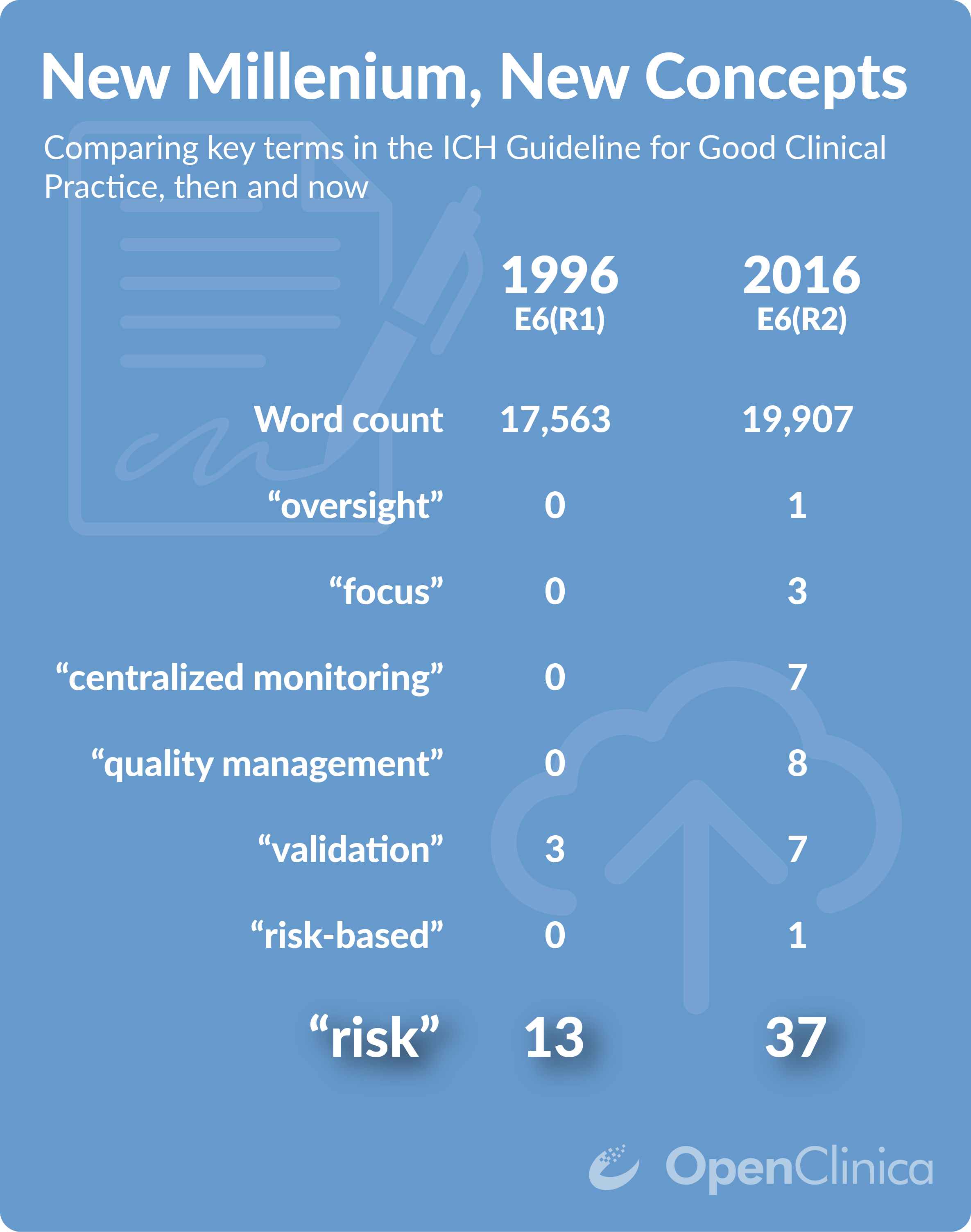


.jpg)


