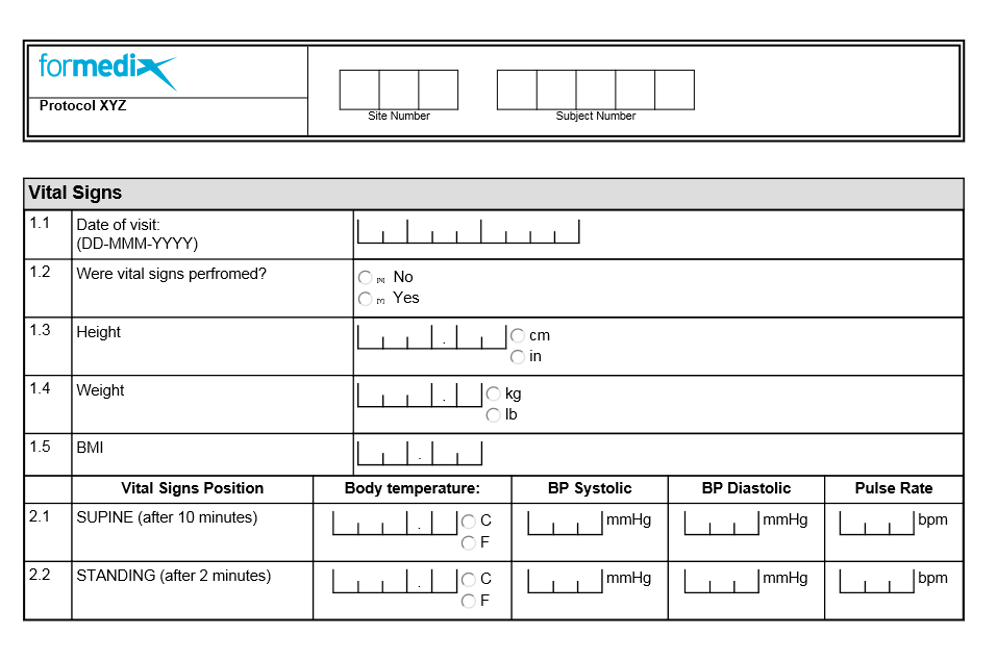
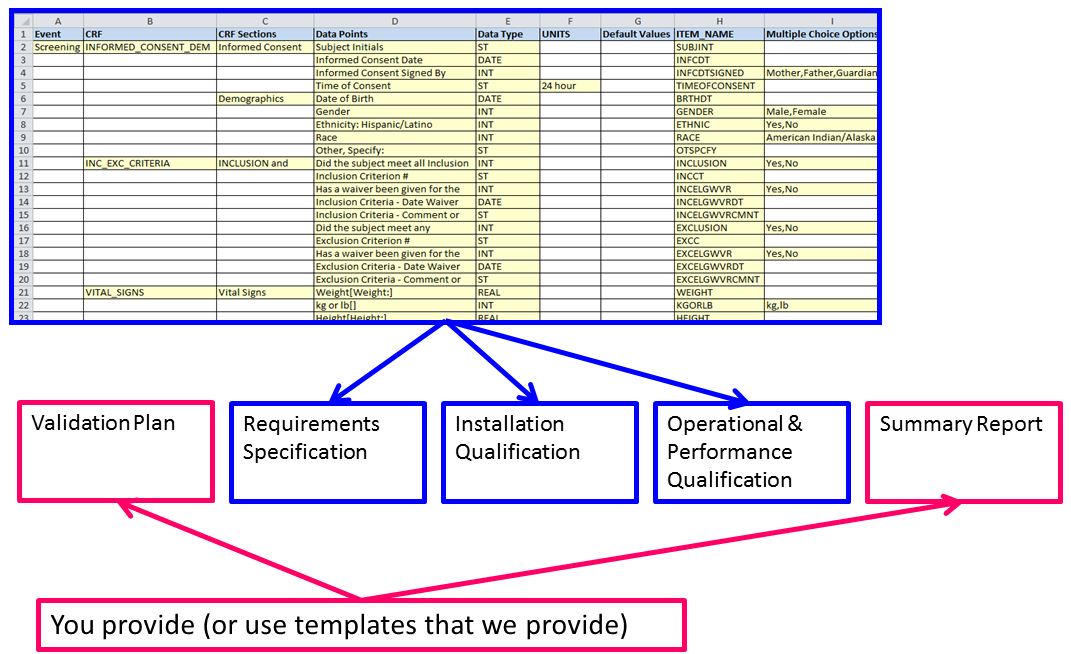
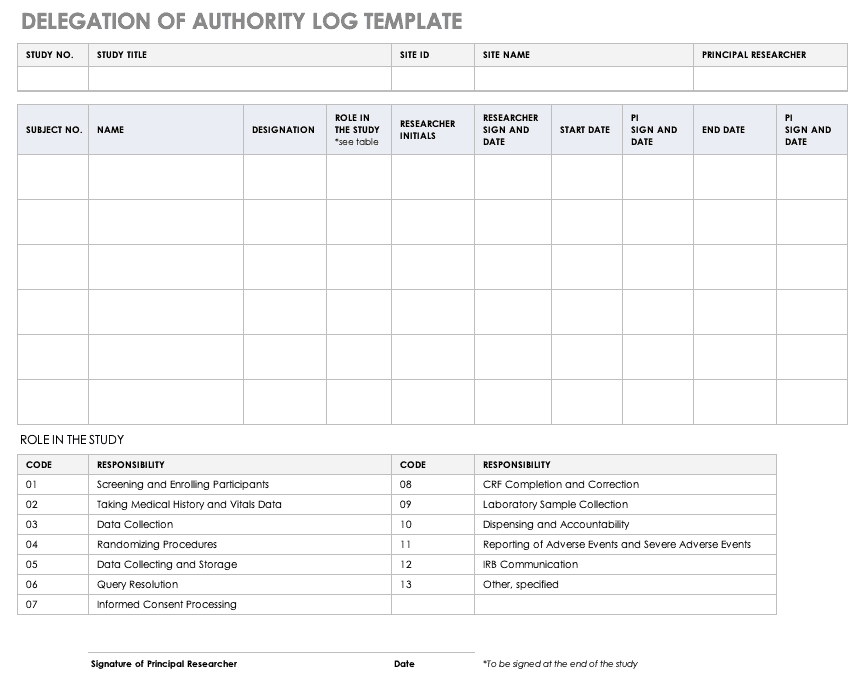
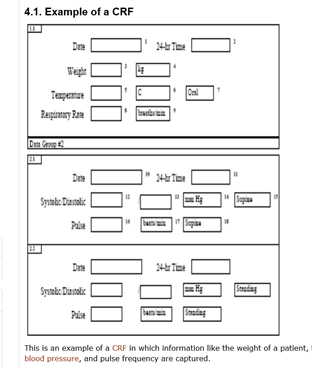
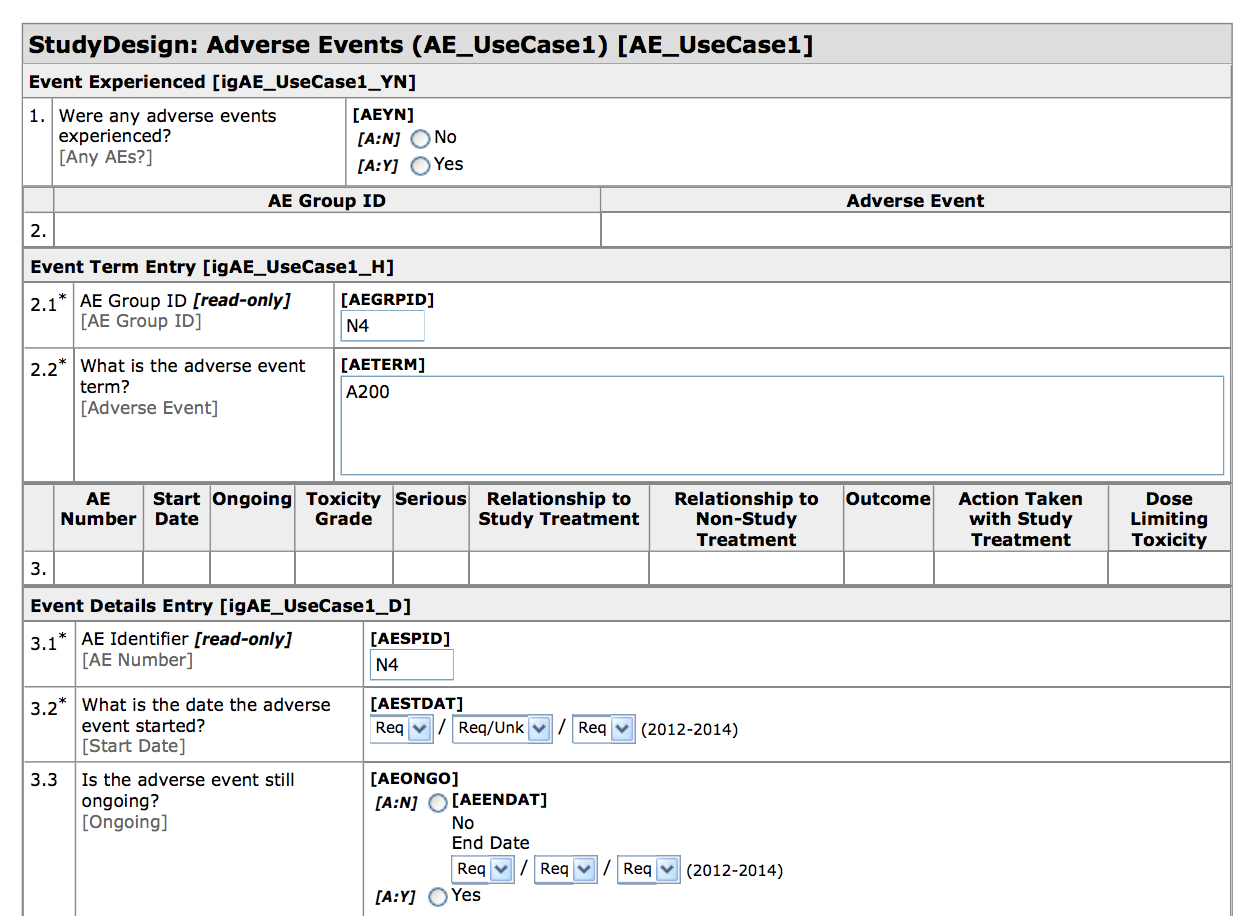
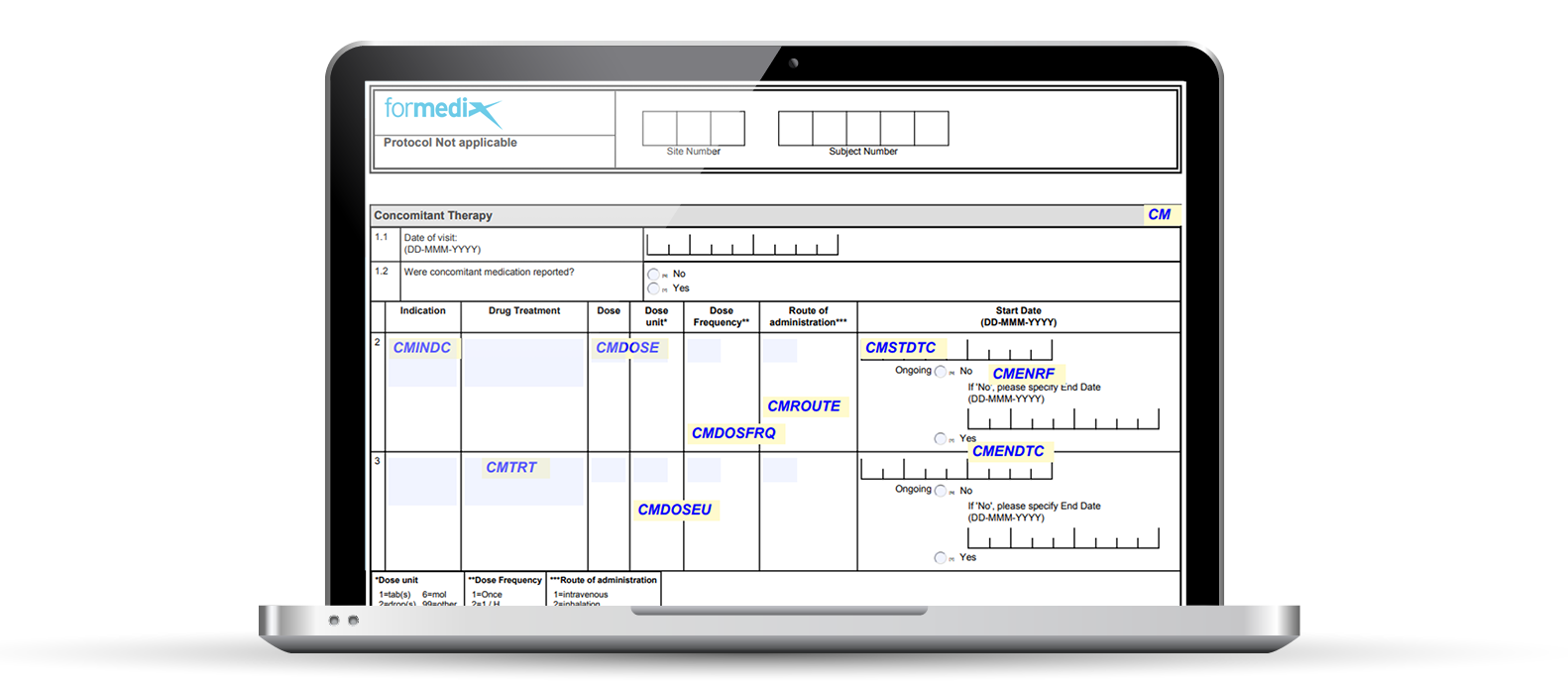
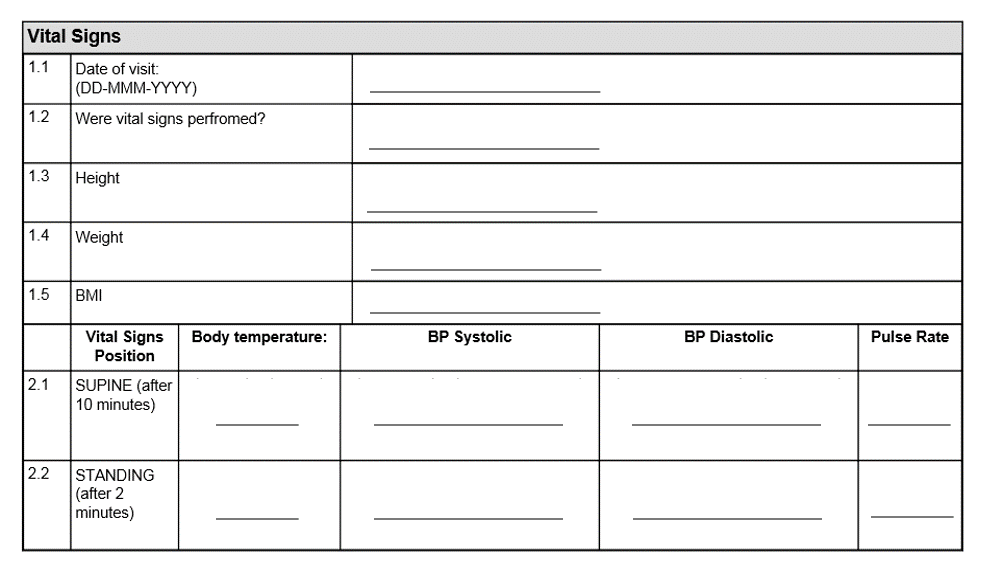
Basics of case report form designing in clinical research Bellary S, Krishnankutty B, Latha M S - Perspect Clin Res

Using Questionnaires in Clinical Research – A Guide through the Data Jungle - Trilogy Writing & Consulting GmbH

Long-term follow-up form. Simple one page CRF for collection of primary... | Download Scientific Diagram

Clinical Trials Support Unit task delegation log. AE, adverse event;... | Download Scientific Diagram

Case Report Form (CRF) for clinical examination 1. CRF to be filled by... | Download Scientific Diagram

Case Report Form Template Clinical Trials (3) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Report template, Study site, Clinical trials