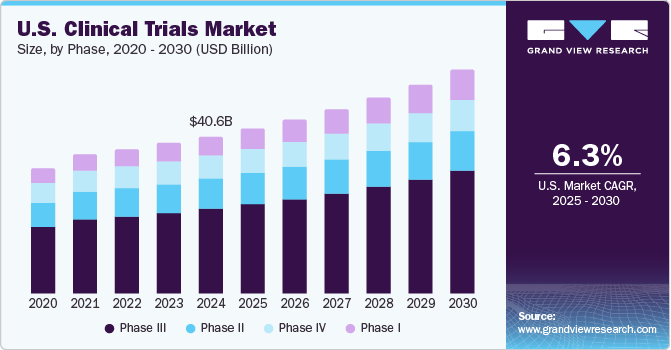
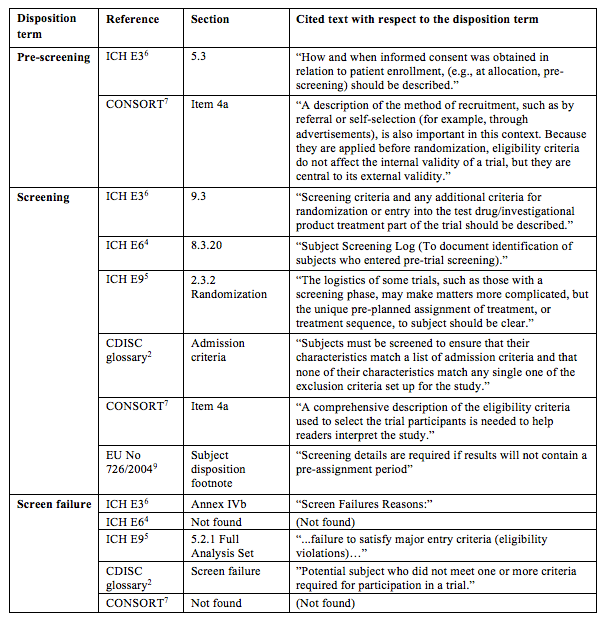
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine

Will clinical trial data disclosure reduce incentives to develop new uses of drugs? | Nature Biotechnology

Clinical Trial Report Template (6) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Report template, Clinical trials, Template google
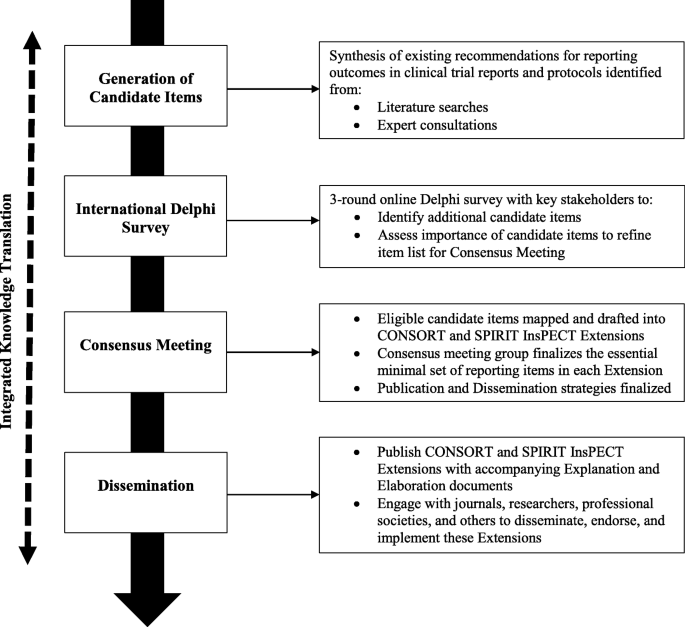
Improving outcome reporting in clinical trial reports and protocols: study protocol for the Instrument for reporting Planned Endpoints in Clinical Trials (InsPECT) | Trials | Full Text
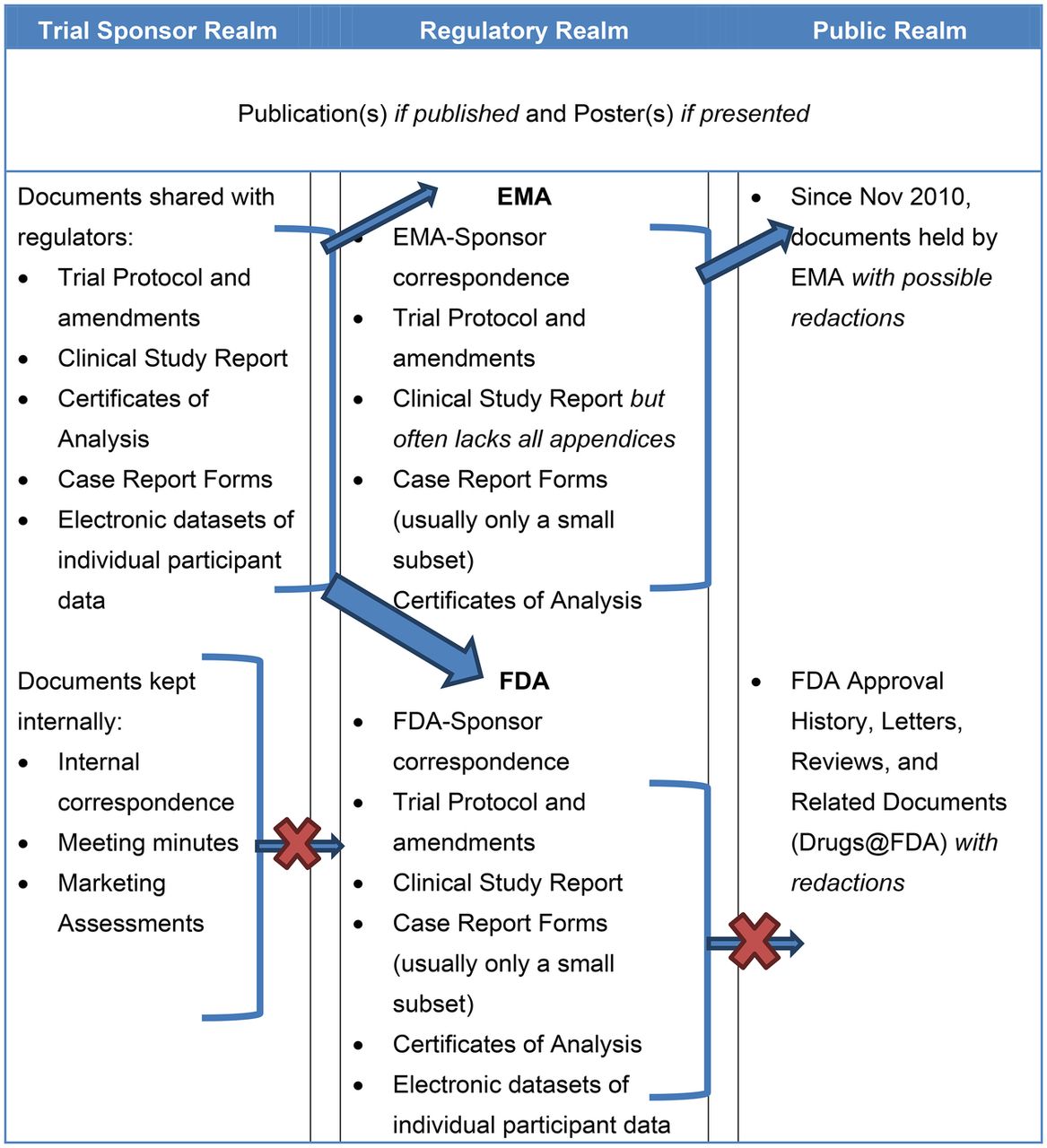
Clinical study reports of randomised controlled trials: an exploratory review of previously confidential industry reports | BMJ Open

Services - Clinical Trial Report Preparation Services from Lucknow Uttar Pradesh India | ID - 3118976
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine

Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension - The Lancet Digital Health