Pharmaceuticals | Free Full-Text | Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trials Office | HTML
Annex 1: CLINICAL TRIAL APPLICATION FORM (CTA) To be completed by Applicants for all Clinical Trials Study Title: Protocol No:

Solve Expiry Labels, DtP, and Timelines for EU 536/2014 Clinical Trials Regulation | Healthcare Packaging

Guidance Notes for Applicants of the Certificate for Clinical Trial on Medical Device - PDF Free Download
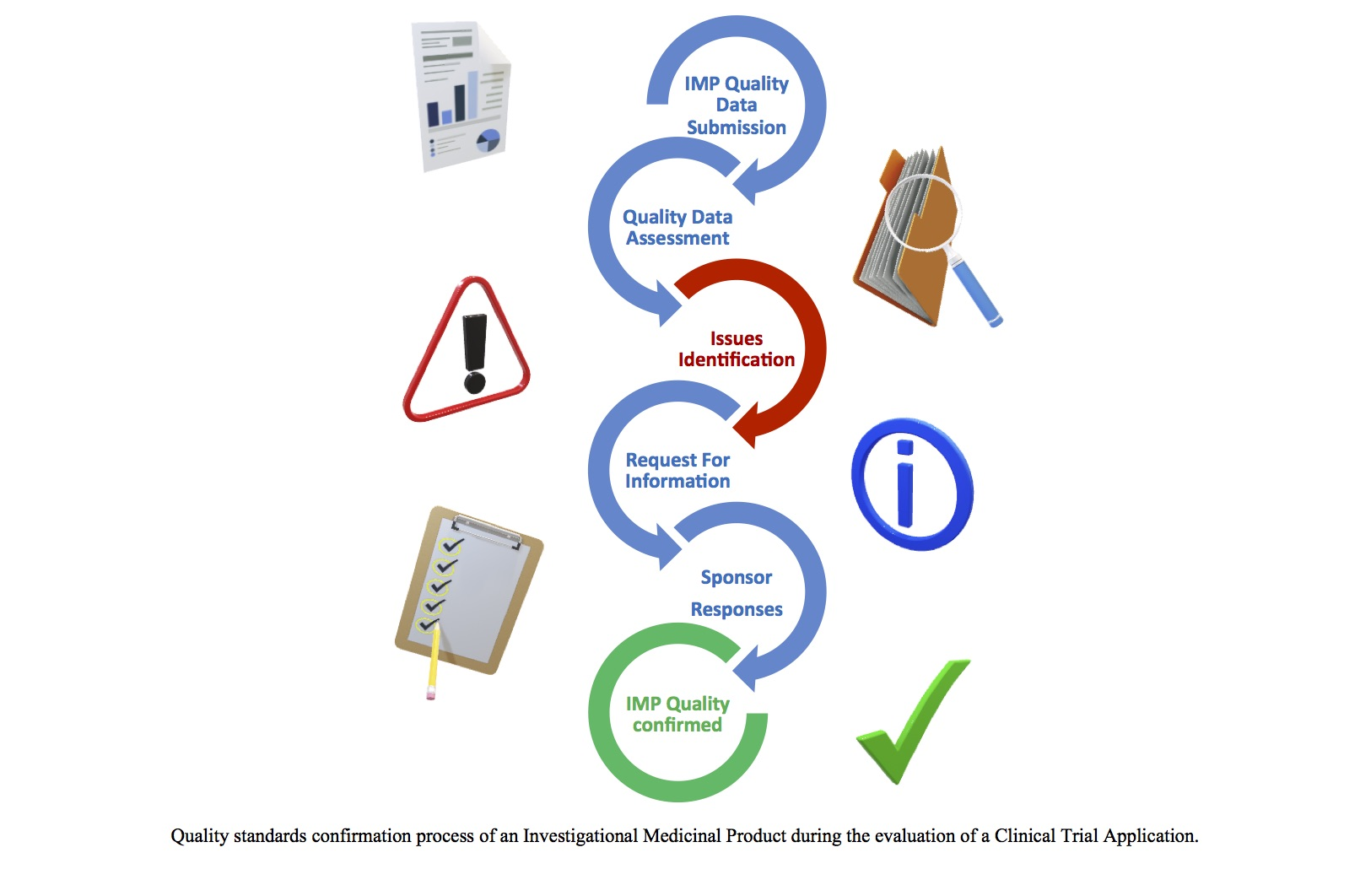
Quality Assessment of Investigational Medicinal Products in COVID-19 Clinical Trials: One Year of Activity at the Clinical Trial

Clinical trials were missing from regulatory documents of extended-release methylphenidate for ADHD in adults: a case study of public documents - Journal of Clinical Epidemiology